THE PROBLEM
At Zimmer and Peacock we develop electrochemical sensors, biosensors and medical diagnostics; something that is rarely mentioned both in the academic or industrial setting is the reference electrode. Often the reference electrode is silver/silver chloride, but the term not often used but which should be accurately applied is pseudo reference electrode.
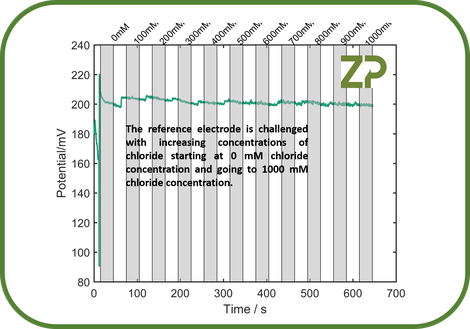
What this means is that the reference electrode is only a reference electrode under certain conditions of stable chloride concentration. In many applications chloride can be considered to be stable and repeatable, for example the chloride concentration in blood, plasma, serum, interstitial fluid etc the chloride concentration is around 135 to 150 mM. In other applications such as urine analysis, water testing, food testing etc, the chloride concentration maybe unknown and maybe a variable. The issue is that a silver/silver chloride reference electrode has a potential that is effected by the sample's chloride concentration, this is shown in the adjacent image.
The effect of having a pseudo reference electrode is that sensors based on amperometry potentiometry, voltammetry, etc many not function as expected if the chloride concentration is unknown and variable.
THE SOLUTION
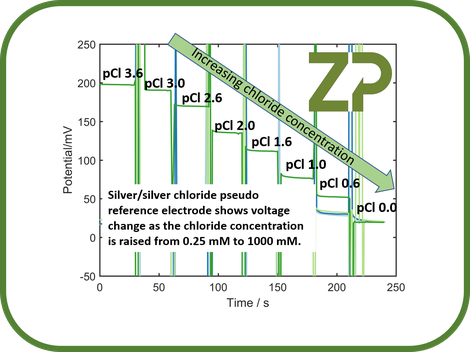
ZP has developed a reference electrode that it can apply to its standard products that is insensitive to changes in chloride concentration, please see adjacent image.