Zimmer & Peacock, in the role as a provider for industry and consumer applications, is centered around electrochemical sensors for various applications. Zimmer & Peacock develops, manufactures and distributes products within healthcare diagnostics, agriculture, aquaculture, food and beverage and
several other markets.
We, at Zimmer & Peacock, take our responsibility towards our clients and consumers very seriously, and therefore we are fully committed to complying with the applicable regulatory requirements, to maintain the effectiveness of the quality management system and to deliver products with the highest standard of
quality, performance, and safety. We design effective processes and a quality management system that is regularly reviewed and improved to ensure compliance with client and regulatory requirements. All personnel at Zimmer & Peacock work to ensure the optimum safety and performance of products and
services, and this is the main driver for continuous improvements within Zimmer & Peacock.

License to ZP eQMS
ZP has an eQMS system as part of our ISO13485 quality system, we can place and organise documents in our eQMS system and we can provide a license per user per month.
150,00 €
Final price excl. shipping costs
Free shipping to the following countries: Show more Show less
- Sold Out

Zimmer and Peacock expect our clients to go to market and in part we are driven by programs whose raison d'etre is to deliver commercially successful products, some of which will be delivered into regulated markets.
Zimmer and Peacock have markets including Europe and North America and so we understand the subtleties of going to market with a CE certificate in Europe, or 510K within the CFR 21 code in the USA. Therefore Zimmer and Peacock by default follow ISO 13485.
Zimmer and Peacock are often the science, engineering or manufacturing team behind our clients products so we advise them that as the regulatory facing and customer facing entity that will have to be ISO 13485 certified.
Within Europe a CE mark may be required on going to market and though this has to ultimately come from the regulatory and customer facing entity, Zimmer and Peacock can prepare the documents so that the signatory to the declaration can sign and provide evidence upon which they were able to make that declaration.
If the route to the US market is via a 510K then Zimmer and Peacock can prepare the documents to demonstrate the equivalence of the new products to products already on the market.

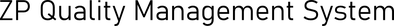
As part of ZP's product development and product management services for biosensors and IVD, we collaborate with our clients around the Quality Management System.
At ZP we know that the science, technology and engineering is only part of the road to commercialisation, and we put as much effort into the documentation and procedures, so that we can lock the product down.
When you endeavour on a biosensor development and manufacturing programme with ZP please know that we are always developing and manufacturing with the current or future QMS in our minds.
If you are looking for a biosensor or IVD developer and manufacturing partner who is as focused on the QMS as the other elements of a programme please don't hesitate to contact ZP.