Detection capability for Invitro Diagnostics

At Zimmer and Peacock we are focused on the development and manufacture of biosensors and medical diagnostics. We have a range of standard products and we are a leading suppler of contract biosensors and IVD development and manufacturing services.
INTRODUCTION
There is much discussion within the market of biosensors and IVDs on how low a concentration of analyte a sensor or IVD can measure. There is often an issue or concern due to a confusion in terms and phrases used to discuss these detection limits.
On this page we are discussing detection limits or detection capability and specifically in the context of sensors and IVDs. There are at least three terms to define when thinking about detection capability these are:
- Limit of blank (LoB) - This is the upper end of the range of signals expected when measuring blank samples.
- Limit of detection (LoD) - This is the concentration of an analyte that can be detected with a yes/no manner, i.e. is it present or not.
- Limit of quantification (LoQ) - This is the minimal detectable concentration of analyte versus a pre-established accuracy goal.
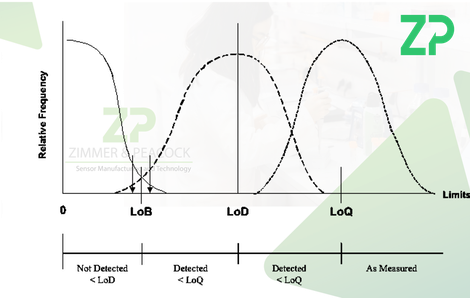
LoB and LoD are based on the inherent performance of the IVD, are calculated from the data in an objectivity statistical manner, whilst LoQ is reflects the underlying reproducibility of the IVD and an accuracy goal set by the developers.