In the context of medical devices software is either the medical device or incorporated into the medical device. For example an app on a smart device able to take a picture of a lateral flow device and apply AI to determine whether the lateral flow strip shows a positive or a negative result is the medical device, whilst the software within an in-vitro diagnostic device can be considered incorporated medical device software,
The requirements for software within the medical field are in IEC62304, which defines the process of classifying the software, developing the software and defines the software's lifecycle
The tenents of IEC62304 are: planning, documentation, verification and traceability, and IEC 62304 applied to medical device software. Software that is used within the manufacturing process is not covered by IEC 62304, and rather software used to manufacture a edical deive is covered under the software valdiations partso ISO13485.
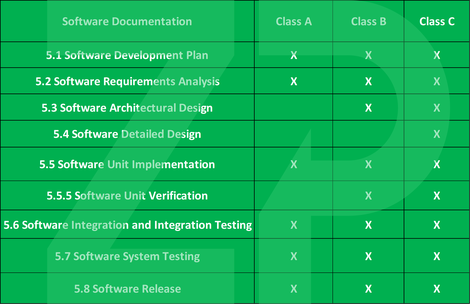
The standard is interesting as it deigned medical device software into the the most stringent of catoegories and it is for the developer to provide a rational explination as to why the software should be moved from Class C to Class B ro Class A.
- Class A - No injusry or damage to health possible
- Class B - Injury possible but not serious
- Class C - Death or seriosu injury possbile
Whether the software is classified Class A, B or C then impacts which specifications are applied to it, with C being the most strict classification.
ABOUT US
ZP is an ISO13485 contract developer and manufacturer of biosensors and IVDs, as part of this we develop software applications to accompany medical diagnostics from lateral flow strips, IVDs, biosensors, transdermal sensors and implantable sensors.