INTRODUCTION
At Zimmer and Peacock we are pragmatists, which means we will choose the best materials for the particular application. When manufacturing a screen printed electrode the choice of ink can be critical or not depending on what the ink will be used for. When it comes to sensors and assays developed on screen printed
In this note we discuss a comparison between two carbon inks from two different suppliers. When choosing an ink there are generally two categories of properties that are that .are considered and one that is overlooked, these are: printability, electrical properties and electrochemical properties.
The printability/rheological properties of an ink and paste are of course in the datasheet of the vendors, and similarly the electrical properties of the ink appear on the datasheets. What is not so much discussed is the electrochemical properties of the ink. Whether the electrochemical properties are important then depends on what kind of assay is being run, whether is is an impedance, amperometric, voltammetric or potentiometric assay. There are no definitive rules, but one could argue that electrochemical properties are important when the assay is voltammetric in nature. If the assay is potentiometric for example then the electrochemical properties are less important. We illustrate this within this article.

We tested two inks from two vendors which were both described as carbon inks: INKS A and INKS B. We were able to print both inks, and when measuring the resistance both had similar conductivity.
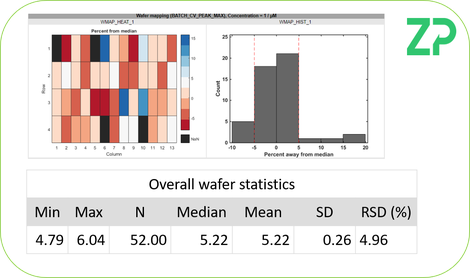
WAFER MAPPING
Once the inks were printed ZP used its wafer mapping technology to assess electrodes fabricated from A and B.
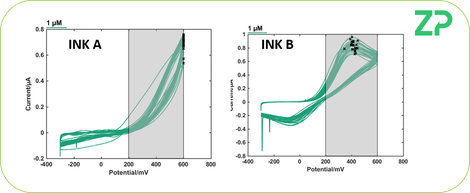
ELECTROCHEMICAL TESTING
Both inks were tested for their electrochemical behavior, and whilst Ink B gave reasonable peaks, ink A did not give the expected electrochemical behavior.
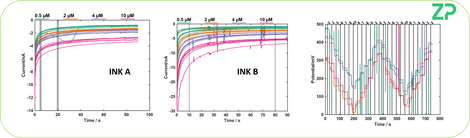
FUNCTIONAL TESTING
Having determined that the electrodes were electrochemically different we could clearly say that for a voltammetric assay ink B performed better than ink A. We then tested both as amperometric and potentiometric sensors. Following these tests we could argue that both ink A and ink B were able to be used in the amperometric and potentiometric inks.
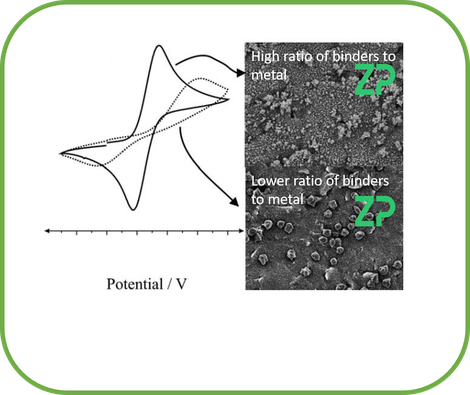
WHY ARE THE INKS DIFFERENT
In the adjacent image we show the route cause of while both inks are conductive one is better for voltammetric assays; the root cause is the ratio of organic binders to metal/metalloid on the surface. Higher the ratio of organic binder to metal/metalloid then the higher the resistance and therefore the more distorted the voltammetry .