At Zimmer and Peacock we are pragmatic industrial electrochemists who see that R and D is just the first step on the route to market.
The biggest issue for academia and the R and D effort is the complaint of irreproducibility of electrodes.
ZP is unique in that we are the only manufacturer who characterizes it's batches of electrodes.
In a recent short study ZP electrodes were analysed against a source of electrodes from another supplier, the researcher remarked that the ZP electrodes were very high grade relative to the competition
At ZP our philosophy is if you don't measure it you can't improve it, and so we always encourage researchers to ask their suppliers for test data on the electrodes, and ask how they characterize each batch of electrodes. If they are unable or unwilling to supply it then one should be very cautious.
TESTING
The independent researchers tested two types of ZP sensors using three electrochemical techniques:
- Open circuit potentiometry (OCP) of the reference electrode vs an external Ag/AgCl reference electrode was recorded to check its stability in a solution with fixed Cl- concentration (0.1M).
- Cyclic voltammetry
- Electrochemical Impedance Spectroscopy.
The electrodes they tested were named P and G, which have the ZP part numbers A-AD-GG-110-N and A-AD-GG-101-N respectively.
RESULTS
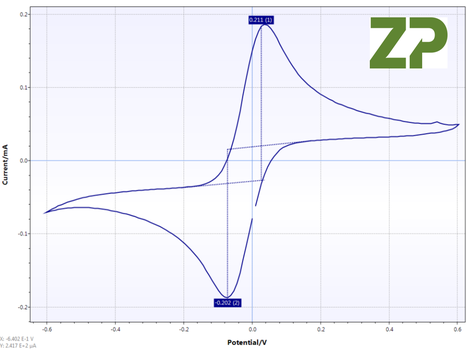
Cyclic Voltammogram
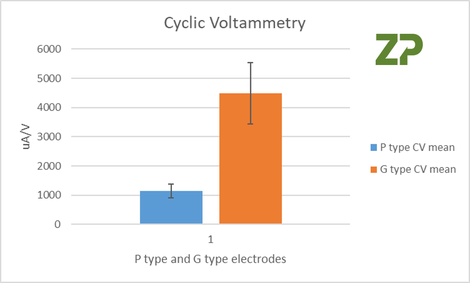
Five P and G electrodes were tested with a known concentration of ferri/ferrocyanide, and the slope from peak to peak of the cyclic voltammetry were measured, the results were for five different electrodes of two different sets. CV% was 20% for P type and 23% for G type.
Impedance Spectroscopy
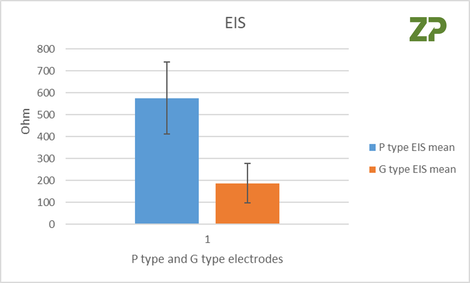
The independent researchers appreciated that cyclic voltammetry was not appropriate if the electrodes were to be used for an analysis where the assay was based on impedance spectroscopy, conductivity or resistivity, therefore on three of each type of electrode P and G they performed EIS analysis. From the Nyquist plot they calculated Rct, whose coefficient of variance was 28 % and 48 % for the P and G type respectively.
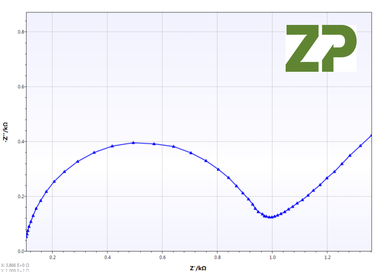
Open circuit potential (OCP)
Finally the scientist wanted to check the referencing potential of the screen printed reference electrode, the P and G Electrodes have the same reference electrode material so there was no difference in the OCP when the reference potential was measured against a lab Ag/AgCl electrode and the screen printed reference electrodes were in 0.1 M chloride solution.
Ten RE were tested and the results are in the table below.
| Mean potential in 0.1 M chloride ve.s lab reference (Ag/AgCl) | Coefficient of variation | delta V in 2 minute window |
| 0.236 V | 1 % | <0.001 V |