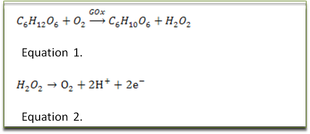Electrochemical Glucose Sensors - the principal
The electrochemical glucose sensor shown here is based on a three-electrode electrochemical cell configuration capable of electro-oxidizing chemical species in solution.
Specificity is achieved by an immobilized layer of the enzyme glucose oxidase (GOx). The system is illustrated in the adjacent Figure; GOx catalyses glucose and oxygen to gluconic acid and hydrogen peroxide see Equation 1.
The arrows closest to the electrode in the figure is the mediator, whose task is to be the charge carrier, shuttling electrons between the catalyst and electrode. The reduced form of the mediator, hydrogen peroxide, is oxidized at the surface of the electrode, releasing electrons into the electrode, following the half- Equation 2.