- Home
- Services
- Newsletter
- Products
- About
- Facility
- Management
- Team
- Scientific Advisory Board
- Corporate Info
- Quality Standards
- Employment and Diversity
- Sales Terms and Conditions
- Shipping
- Group
- Analog Devices Alliance Partner
- Client-centric philosophy
- Biosensor Research Project
- Masters Research Program - SSI
- UN Sustainable Development Goals
- NDA policy
- ZP Culture
- News and Media
- Contact/Support
- Knowledge Base
- Store
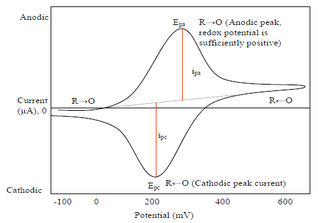
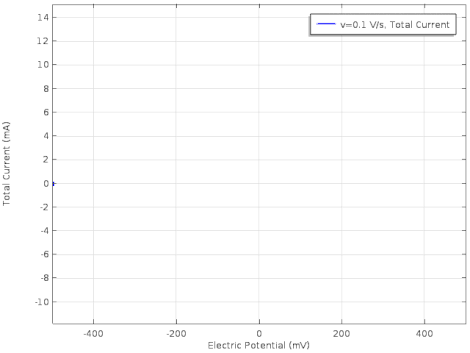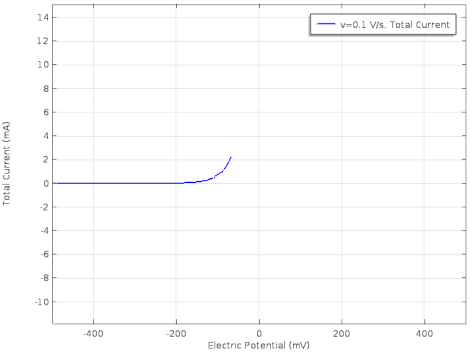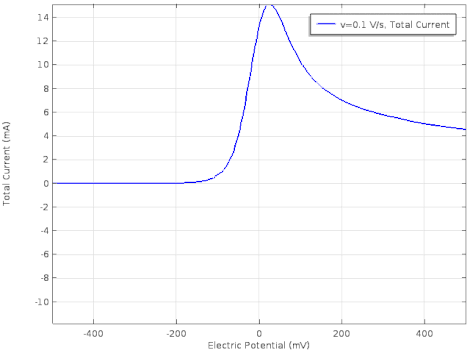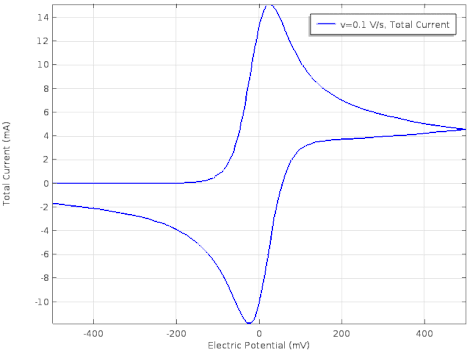
Cyclic voltammetry
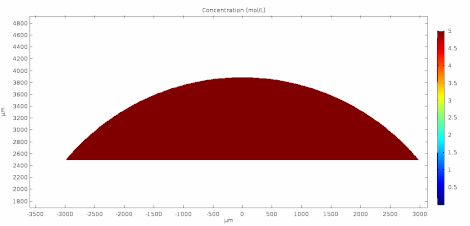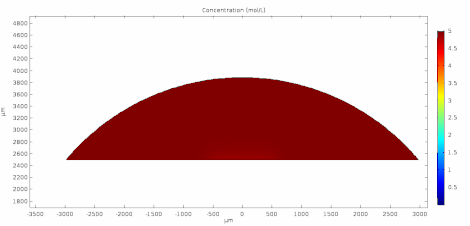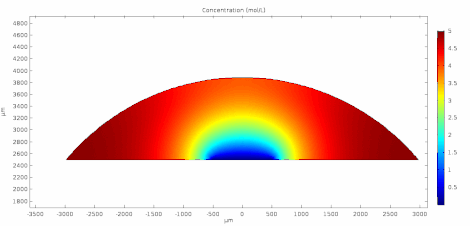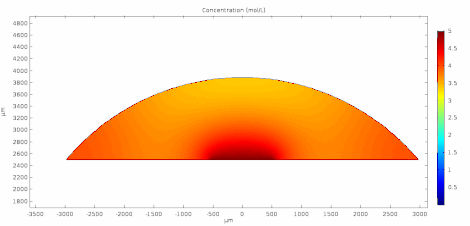
Cyclic voltammetry (CV) is a form of amperometry where the controlled voltage is linearly swept from one value to another, and then back again, resulting in a current as a function of voltage and scan rate. The resulting voltammogram provides important information about the electrochemical reactions occurring at the WE, and the chemical transport kinetics. An exemplary voltammogram of a reversible Fe(III)(CN)6|3-/ Fe(II)(CN)64- couple is provided in the Figure opposite. It starts out at -100 mV relative to the RE. Past 0 mV the electron current n becomes positive, implying that an oxidative electrochemical reaction is happening at the WE. As the voltage increases, the current intensifies until a peak, the anodic peak, is reached. At this point, the oxidation is happening at its fastest possible rate due to the concentration gradient in the close vicinity of the electrode is able to feed the oxidation process with reactants. As the voltage increases the supply of reactants becomes diffusion limited and the current drops to a lower and sometimes constant value. Reversing the sweep results in the reduction of the oxidized species, and a cathodic peak is recorded
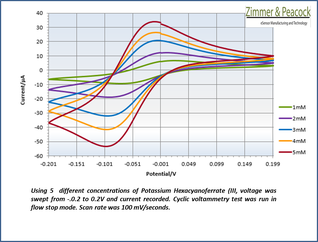
Practical example
The adjacent data was gathered on milli-molar solutions of potassium ferricyanide in 100 mM potassium chloride solution. The sensor used to gather this particular data is in the link.