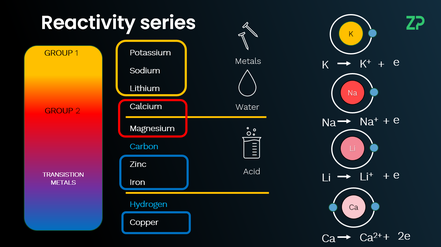
Reactivity Series
In this video we explore the reactivity series of metals, ranking them based on their reactions with water and acids. We'll break down how metals lose electrons to form positive ions and explain key displacement reactions and use the series to predict metal reactivity. From explosive potassium reactions to inert copper, this lesson makes chemistry clear and practical. Watch to deepen your understanding of metal reactivity.
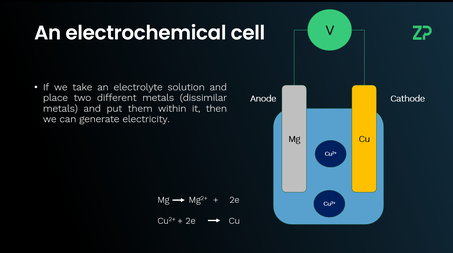
Cells and batteries
This video explains the difference between cells and batteries, electrochemical cells, and voltage potential. A cell consists of two different metals in an electrolyte, generating voltage. Connecting multiple cells in series creates a battery. The reactivity series helps predict voltages from metals positions in the series - farther apart, like magnesium and copper, produce higher voltage than closer ones, like magnesium and zinc. It also covers rechargeable (e.g., lead acid, lithium-ion) and non-rechargeable batteries (e.g., AA, AAA). Lead acid batteries start gasoline cars, while lithium-ion batteries power phones and electric vehicles. Batteries are essential in daily life, from household devices to transportation.