Scientists in the lab, be it biologists and chemists, love to measure their molecules and assays by optical spectroscopy method, be it: UV-VIS absorption spectroscopy, infrared, surface plasmon resonance, fluorescence etc.
The fundamental issue with these lab techniques is that don't always translate when you need to make real world sensors and assays, especially where parameters such as low cost, quantification etc come into play.
If we take a lesson from history we should consider the home glucose test used by diabetics. When these products first came to market they were optical assays, but were quickly replaced by electrochemical assays.
Why were the first glucose home use meters optical? The answer is because the scientists and engineers developing them were used to optical assays, and so they of course they went with what they knew. The issue is that an optically based assay often requires a sample with a high transparency; but high transparency is an immediate issue when you are trying to analyse real-world samples such as whole blood. When people discovered that they could get a glucose signal in whole blood using screen printed electronics/electrodes the market very quickly moved over to electrochemistry. This move from optical assays to electrochemistry by the glucose detection market was not because the scientists and engineers loved electrochemistry, but it was because it offered a much lower cost detection strategy with a lot less sample workup.
What is not widely appreciated is if you can record a spectrum such as a UV-VIS spectrum then you are almost guaranteed to be able to obtain an equivalent electrochemical voltammetry spectrum. For example if you have a molecule of interest and you can measure the UV-VIS spectrum, then you can record the equivalent electrochemical spectrum, this is shown in the adjacent image.
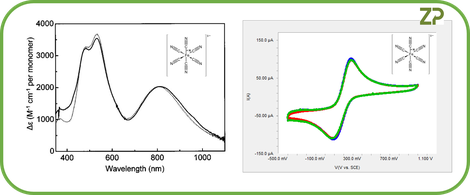
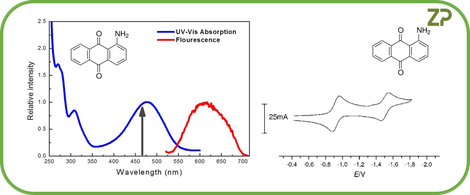
The existence of an electrochemical spectrum for every optical spectrum, in ZP's experience, is universally true, we show another example in the adjacent image.
If developing low cost real-world solutions at the right price is important to you then, there is a fundamental/quantum reason when electrochemistry 'beats' optical techniques and it comes down to whether it is easier to measure an electron or a photon. An electron has mass and charge, whilst a photon has no mass and no charge. Any electronics engineer can measure electrons, it is called electrical current, but it takes more effort to measure something with no mass or charge, such as a photon. This quantum difference between a photon and an electron means that the electronics needed to measure an optical system are more expensive than the electron system.
As discussed an optical system often requires transparent clean sample, free from chunks and lumps, but also an optical system also requires shielding from the ambient light. We are surrounded by light and so special measures have to be taken to shield the sample from the light. If we return to the example of the glucose strip for diabetes, there is no shielding of the assay in place, and this is because electrochemistry doesn't 'care about' light. This reduces the cost of electrochemical products as the engineers don't have to design special shielding for the assay.
Zimmer and Peacock is a unique company in that we have a full range of analytical techniques from UV-Vis spectrometers, UV-VIS-HPLC, plate readers, potentiostats etc, and so we are very used to developing and validating assays using the spectrum of analytical techniques. If you have any questions regarding this article please don't hesitate to contact us.