If you are reading this page you have probably stumbled across it because you are confused by the sign conventions in electrochemistry. Firstly you are not alone, so many people who are trying to look at electrochemical data are slightly bewildered, including us electrochemists.
The reason for this confusion is because whether you are interested in: electronalaysis, biosensor, batteries, fuel cells, capacitors, electrolysis etc you have a different perspective on whether a current and voltage should be displayed with a positive or negative sign. We had an enquiry recently based on this confusion.
THE ENQUIRY
We had a recent enquiry where two labs were working on the same programme but gave very different looking data, see Figure 1.0.0.
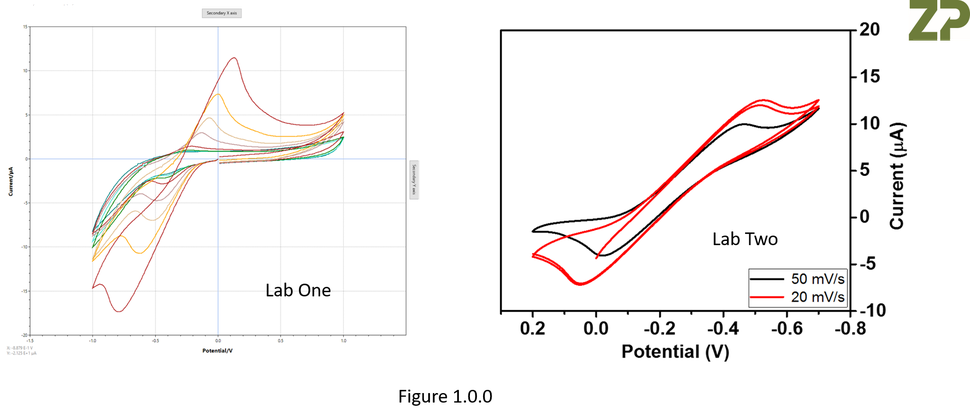

In the data above both labs plot voltage on the x-axis and current on the y-axis, but LAB ONE plotted negative voltages to the left of the zero point and positive to the right hand side of the zero point, whilst LAB TWO had it in the opposite direction, see Figure 1.0.1.
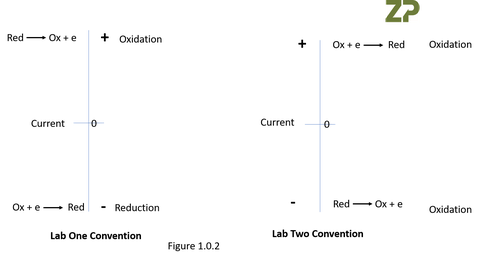
When Zimmer and Peacock looked at the data in Figure 1.0.0.a little deeper we also saw a second less obvious discrepancy betwen the labs; we realised that they had opposite conventions on the sign of the current.
In LAB ONE they showed a current with a positive sign for the oxidation of a molecule and for the reduction of the molecule they had a negative sign on the current, whilst in LAB TWO they had an opposite convention in that LAB TWO showed the current for the reduction of the molecule with a positive sign and the oxidation of the molecule as a current with a negative sign, we have illustrated this in Figure 1.0.2.
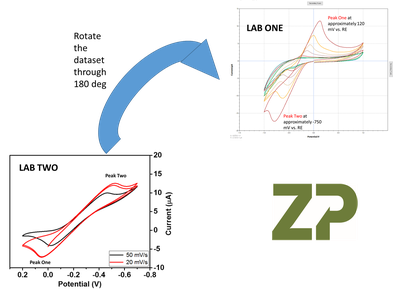
Though this may sound very unsatisfactory to make an easier comparison of the data it was necessary to rotte it through 180 deg. This does feel deep unsatisfactory a process when you want simply compare data.
CONCLUSION
At Zimmer and Peacock we are electrochemists and we are sometimes confused by which convention is being used , our advice is if you are confused please feel free to send us an enquiry regarding the data you are trying to interpret by clicking the link below.